Histology
The following techniques take days to weeks to perform. The core rooms provide designated laboratory space for isotope and sterile methods as well as a dark room, x-ray film cassettes and optimizing protocols for these techniques. The core offers equipment and instruments that are used to process samples for these techniques. Images below are an example of visualing areas of interest with these techniques.
The type of training available is:
- Operator Assisted – working with the researcher on the bench one on one throughout the assay or on a one time basis until trained and the Operator performs the assay Independently.
- Independent – researcher works on their own inside the core room after demonstrating competency with equipment and laboratory practice.
- Consulting – regarding any aspect of the technique or protocol the researcher needs assistance throughout the experiment, whether one time meeting or throughout the protocol including troubleshooting or optimizing a protocol with the operator.
 Immunohistochemistry (IHC) refers to the process of detecting antigens (e.g., proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues. IHC takes its name from the roots “immuno,” in reference to antibodies used in the procedure, and “histo,” meaning tissue.
Immunohistochemistry (IHC) refers to the process of detecting antigens (e.g., proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues. IHC takes its name from the roots “immuno,” in reference to antibodies used in the procedure, and “histo,” meaning tissue.
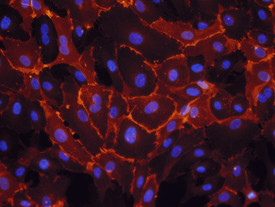 Immunocytochemistry (ICC) is a common laboratory technique that is used to anatomically localize presence of a specific protein or antigen in cells by use of a specific primary antibody that binds to it. The primary antibody allows visualization of the protein under a fluorescence microscope when it is bound by a secondary antibody that has a conjugated fluorophore. ICC allows researchers to evaluate whether or not cells in a particular sample express the antigen in question. In cases where an immunopositive signal is found, ICC also allows researchers to determine which sub-cellular compartments are expressing the antigen.
Immunocytochemistry (ICC) is a common laboratory technique that is used to anatomically localize presence of a specific protein or antigen in cells by use of a specific primary antibody that binds to it. The primary antibody allows visualization of the protein under a fluorescence microscope when it is bound by a secondary antibody that has a conjugated fluorophore. ICC allows researchers to evaluate whether or not cells in a particular sample express the antigen in question. In cases where an immunopositive signal is found, ICC also allows researchers to determine which sub-cellular compartments are expressing the antigen.
Immunocytochemistry differs from immunohistochemistry in that the former is performed on samples of intact cells that have had most, if not all, of their surrounding extracellular matrix removed. This includes cells grown within a culture, deposited from suspension, or taken from a smear. In contrast, immunohistochemical samples are sections of biological tissue, where each cell is surrounded by tissue architecture and other cells normally found in the intact tissue. Immunocytochemistry is a technique used to assess the presence of a specific protein or antigen in cells (cultured cells, cell suspensions) by use of a specific antibody, which binds to it, thereby allowing visualization and examination under a microscope. It is a valuable tool for the determination of cellular contents from individual cells. Samples that can be analyzed include blood smears, aspirates, swabs, cultured cells, and cell suspensions.
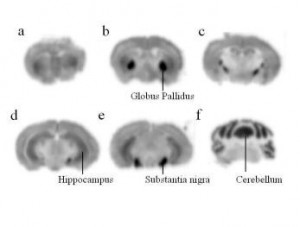 Autoradiography is the process of taking a type of picture, called an autoradiograph which shows the relative concentration of radioactive material present with tissues such as chemical receptors, their distribution within tissues, and their function, cellular and tissue structures and how radioactive materials are absorbed and distributed. Study RNA and DNA as well as the phases of cell development and function. It can also be used to isolate patial sequences of RNA and DNA for study, especially in viruses. Non-radioactive labels may also be used to label your target proteins.
Autoradiography is the process of taking a type of picture, called an autoradiograph which shows the relative concentration of radioactive material present with tissues such as chemical receptors, their distribution within tissues, and their function, cellular and tissue structures and how radioactive materials are absorbed and distributed. Study RNA and DNA as well as the phases of cell development and function. It can also be used to isolate patial sequences of RNA and DNA for study, especially in viruses. Non-radioactive labels may also be used to label your target proteins.
 In-Situ Hybridization or On-site hybridization is often used to see where certain macromolecules reside inside a tissue. This process can be used to localize gene expression at the cyctological level. Markers used to detect target molecule include isotopes, digoxigenin (DIG) pictured and fluorescent.
In-Situ Hybridization or On-site hybridization is often used to see where certain macromolecules reside inside a tissue. This process can be used to localize gene expression at the cyctological level. Markers used to detect target molecule include isotopes, digoxigenin (DIG) pictured and fluorescent.
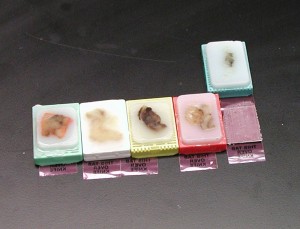 Paraffin Infiltration and Embedding
Paraffin Infiltration and Embedding
Paraffin embedding is used to prepare the tissue specimen so it does not shred upon slicing. The tissue specimen is infused with paraffin (wax) and placed within a mold to which more melted paraffin is added. The result is a small cube of wax with the paraffin-filled tissue specimen within it. Tissue embedding is routinely used to prepare tissue biopsies for examination.
This process involves three steps including (see Specialized equipment of description of equipment used):
1) Infiltration – requiring a protocol for fixation, dehydration, clearing and wax infiltration of tissue performed in the STP 120. PSC 863/841
2) Embedding (Pictured above) – placing the tissue into a paraffin block using the HistoStar 4. PSC 861/843
 3) Sectioning – cutting the tissue block on a Rotary Microtome, mounting on slides using a water bath and heating plate. PSC 921
3) Sectioning – cutting the tissue block on a Rotary Microtome, mounting on slides using a water bath and heating plate. PSC 921