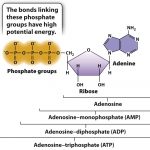
All cells require ATP to perform any action where an input of energy is needed. ATP stands for Adenosine triphosphate and is well known as the “energy currency” of all cells. We relate it to currency because ATP is not the energy itself, rather it is a molecule with a high energy value stored in its chemical bonds. Its role in metabolism is similar to the way a dollar bill represents purchasing power. The more ATP a cell contains, the more energy it has readily available for use when needed. ATP is composed of a triphosphate group (a chain of three phosphates) and adenosine (an adenine attached to a five-carbon ribose sugar). Below is a picture representing its chemical structure.
Because its energy value comes from its chemical bonds, ATP is considered a form of chemical potential energy. The reason it has such a high energy value relative to other molecules is primarily because of its unstable phosphate group. The bonds are unstable because phosphates are negatively charged and try to repel each other, but they are held together by weak hydrogen bonds. The fact that the phosphates are naturally repelling each other due to their opposing charges and are held together by weak hydrogen bonds make it easy for a cell to break the bond in a hydrolysis reaction, and in doing so will transform its stored chemical energy into mechanical energy that will be used to drive most cellular processes.
Though one molecule of ATP is structurally identical to others, the energy output from the hydrolysis of ATP can vary. The maximum output from the hydrolysis of ATP within a living cell can be up to -57kj/mol, however environmental conditions within the cell like PH and Magnesium ion (Mg2+) concentration can change this value. Note the negative sign implies energy is released from the reaction, so it is classified as exergonic. The second law of thermodynamics states that energy will be inevitably lost in the reaction in the form of heat, so it is impossible for the reaction to be 100% efficient. To compensate for this loss ATP catabolisms are coupled with chemical reactions of other forms, namely with redox reactions. The synthesis reaction of ATP in the form of a chemical equation is ADP+Pi+free energy–>ATP + H2O. ADP stands for Adenosine diphosphate and is a more stable molecule. This reaction is reversible, and therefore adding a Pi group to ADP with enough added energy will yield ATP. Clearly, without ATP, life on earth would not be possible, so understanding the role of ATP in energy metabolism is critical to understand life’s processes.
References
ATP: Adenosine Triphosphate. (2017). Retrieved April 24, 2017, from Boundless Biology Boundless: Retrieved from https://www.boundless.com/biology/textbooks/boundless-biology-textbook/metabolism-6/atp-adenosine-triphosphate-71/atp-adenosine-triphosphate-349-12938/
Biology: How life works (2nd ed.). (2017). In B. A. Berry Andrew, Biology: How life works (2nd ed.) (p.115-123, CH 6). McGraw-Hill, NY: W. H. Freeman