Leeza-Marie Williams
Endomembrane system:
The endomembrane system consists of a group of membrane bound organelles that are found in eukaryotes. These organelles work together to modify, package, and transport lipids and proteins. It is important to know that mitochondria, chloroplasts, and peroxisomes are not included in the endomembrane system.
The endoplasmic reticulum (ER) consists of a rough ER and a smooth ER which are important in the modification process of proteins and the synthesis of lipids. Structurally, the ER consists of membranous tubules that have a hollow space called lumen, and flattened sacks.
Rough ER have ribosomes attached to its cytoplasmic surface which makes protein chains that are sent into the lumen. The protein that is not transferred fully are anchored in the membrane. Proteins inside of the membrane fold and are modified with the addition of carbohydrate side chains. Modified proteins are incorporated into the membrane of the ER, other organelles, or secreted from the cell. Proteins that are sent out of the cell are first sent to the Golgi apparatus. Phospholipids that are manufactured in the rough ER are also transported out via vesicles. Smooth ER have several functions including synthesizing carbohydrates, lipids, and steroids, detoxification, and storing calcium.
As mentioned previously, proteins that bud off of the ER are sent to the Golgi apparatus where there are sorted, tagged, packaged, and distributed to the right location. The Golgi apparatus has a cis face where it receives protein and a trans face where protein is sent away from the apparatus. A fusion occurs between transport vesicles of the ER and the cis face of the Golgi apparatus where the contents within the transport vesicles are emptied into the lumen of the Golgi apparatus. Further modification may occur to proteins and lipids within the Golgi apparatus. For example, the addition or removal of short chained molecules of sugar or phosphate groups may occur. Modified proteins are sorted based on their amino acid sequences and chemical tags and are packaged into vesicles that bud away from the Golgi apparatus at its trans face. Some of the vesicles that budded off of the Golgi apparatus are sent to the lysosome or vacuole or fuse with the plasma membrane to secrete proteins outside of the cell.
The lysosome contains digestive enzymes that breaks down structures to reuse their molecules. Lysosomes also have the ability to digest foreign particles. For example, macrophages are a class of white blood cells that folds inward to engulf pathogens. Once the pathogen is contained, it forms a phagosome when it is pinched off from the plasma membrane. The phagosome and the lysosome fuse to form a compartment consisting of digestive enzymes that destroy the pathogen. Vacuoles are an alternative to lysosomes in plants which stores water, wastes, isolates pathogens, and breaks down macromolecules.
Mitochondria:
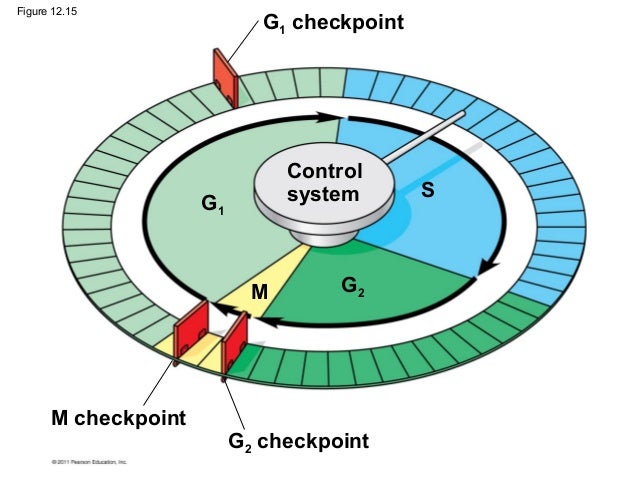
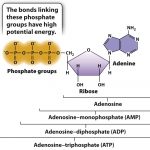
Mitochondrion are different from most other organelles because it has its own circular DNA, similar to the DNA of prokaryotes, and reproduces independently of the cell. Therefore, mitochondrion are endosymbiotic. Mitochondria convert oxygen and nutrients into adenosine triphosphate (ATP). ATP is the chemical energy “currency” of the cell that powers the cell’s metabolic activities. This process is called aerobic respiration and is the reason animals breathe oxygen. Without mitochondria, more complex animals, like humans, would likely not exist because their cells would only be able to obtain energy with the absence of oxygen or anaerobic respiration which is less efficient than aerobic respiration.
Most mitochondria are oblong organelles between 1 and 10 micrometers in length. Both the inner and outer mitochondrial membranes resemble the plasma membrane in molecular structure. Each are 60 to 70 Ǻ thick and are composed of two layers of phospholipid molecules in between two layers of protein molecules. The outer and the inner membranes contain specific channels for molecules to transport through them. The two membranes may be connected at adhesion sites. The outer and inner mitochondrial membranes are separated from each other by a narrow space called the intermembrane space.
The outer membrane is freely permeable to most small molecules. such as ions, ATP, ADP, and nutrient molecules because it consists of transmembrane channels formed by the protein, porin. The inner membrane is freely permeable only to oxygen, carbon dioxide, and water. Its structure is highly complex, including all of the complexes of the electron transport system, the ATP synthetase complex, and transport proteins. The wrinkles, or folds within the innermembrane are organized into layers called the cristae which divides the mitochondrion into compartments running perpendicular to the long axis of the rod shaped mitochondrion. The cristae greatly increase the total surface area of the inner membrane which provides enough space for the previously mentioned structures to fit inside of the mitochondria. The matrix contains enzymes that are responsible for the citric acid cycle reactions. The matrix also contains dissolved oxygen, water, carbon dioxide, the recyclable intermediates that serve as energy shuttles. An electron carrier is a molecule that accepts an electron from one molecule and transports it into another, this movement of electrons by carriers is known as the electron transport chain. Carriers can exist in either an oxidized or reduced form, in an oxidized form the carrier is available to receive additional electrons, in a reduced form the carrier is actively carrying electrons.
Chloroplasts:
The process of photosynthesis is conducted using chloroplasts which are used to convert light energy into chemical energy. Since chloroplasts are involved with storing energy and synthesizing metabolic materials, they are categorized as a type of plastid. Various types of plastids are required depending upon the amount of light surrounds the leaf as it grows. Leucoplasts are used to synthesize starch, oil, and protein. Chromoplasts that are yellow to red synthesize carotenoids. Green chloroplasts contain chlorophyll a and chlorophyll b which are involved with absorbing light energy required for the process of photosynthesis.
To describe the structure of the chloroplast, it is enclosed in a double membrane consisting of two layers within the membrane called the intermembrane space. In terms of permeability, the outer portion of the double membrane is more permeable than the inner portion of the membrane. The stroma comprises of most of the chloroplast volume by containing a semi fluid material consisting of enzymes. Like mitochondria, chloroplast have their own DNA, ribosomes, and RNA which are found in the stroma. More complex plants have internal membranes (lamellae) with stacks (granum) of closed hollow disks (thylakoids). Lumens or internal spaces allow for the connection of thylakoids. Within the thylakoids are embedded chlorophyll molecules. Light travels in photons and are absorbed by molecules of chlorophyll. Once absorbed, electrons are emitted and hydrogen ions are propelled around the thylakoid stack. When hydrogen ions surround the thylakoid stack, formation of an electrochemical gradient occurs and the stroma produces ATP. Light independent reactions that involve carbon fixation occur in the stroma whereby low energy carbon dioxide molecules transforms into glucose, a high energy compound.
Cytoskeleton:
Eukaryotes possess three types of protein fibers in their cytoskeleton: microfilaments, intermediate filaments, and microtubules. Microfilaments are the narrowest type of protein fibers with a diameter of 7nm consisting of actin filaments which are linked monomers of protein that resembles the structure of a DNA’s double helix. Within microfilaments are actin subunits. Actin filaments have two structurally different ends that allow them to have directionality. They provide direction when myosin or motor proteins are needed to move around in the cell. Actin filaments also function as a means to enable the cell to transport protein containing vesicles and organelles. Actin and myosin work together in muscle cells to form sarcomeres which are structures of overlapping filaments. When sarcomeres slide past each other, the muscles within our body contracts. Since actin filaments have the ability to assemble and disassemble quickly, it plays a vital role in cell movement.
Intermediate filaments consist of multiple wound strands of fibrous proteins and are the in between the size of microfilaments and microtubules with a diameter of 8 -10 nm. Intermediate filaments differ than actin filaments in that intermediate filaments are more permanent and are specialized to handle tension in order to maintain the shape of the cell. They also serve to anchor the nucleus and other organelles in a specific location.
Contrary to their name, microtubules have the largest diameter at 25nm of all three types of cytoskeleton fibers. Microtubules consist of an arrangement of straw like α-tubulin and β-tubulin subunits. Microtubules are similar to actin filaments in that they have the ability to grow and shrink whenever tubulin proteins are added or removed. Since both actin filaments and microtubules have directionality, they both resist compression forces due to possessing two ends that are structurally different from one another. Without microtubules, motor proteins like kinesins and dyneins would not be able to transport vesicles within the cell. Microtubules are also important in cell division whereby microtubules assemble into spindles that pulls the chromosomes apart.
Protein trafficking:
Membrane bounded transport vesicles transport proteins embedded in the rough ER to the Golgi apparatus. The Golgi apparatus consists of cisternae which are flattened membrane sacs. Translation and modifications of proteins occurs in the rough ER. Modifications are completed in the Golgi apparatus as proteins pass through the complex to ensure that the proteins reach the correct final destination. Each region of the Golgi apparatus contains different enzymes depending on how it plans to modify proteins. As mentioned previously under the topic about the endomembrane system, proteins travel from the cis to the trans regions of the Golgi apparatus where they are then released to the lysosomes or vacuoles or outside of the cell.
Cell signaling:
There are four general categories of chemical signaling: paracrine signaling, autocrine signaling, endocrine signaling, and signaling by direct contact. The main difference between these types is the distance that the signal travels through the organism to reach the target cell.
Paracrine signaling occurs between cells when they are relatively close to one another. In paracrine signaling the cells communicate through releasing ligands that can diffuse through the space between the cells. Paracrine is critical to the process of development and to synaptic signaling of nerve cells transmitting signals.
Autocrine signaling involves a cell releasing a ligand that binds to the receptors on its own surface or inside of the cell. It is beneficial for enabling cells to reinforce their correct identities during development. Autocrine signaling is also beneficial to the spread of cancer from its original site to other parts of the body, metastasis.
The process of signals that are produced by specialized cells and released into the bloodstream where they are carried to target cells is called endocrine signaling. Endocrine signaling transmit signals over long distances. Hormones are signals that are produced in one part of the body and are carried to other targets via circulation.
Signaling by direct contact occurs when two cells bind to each other because they have complementary proteins on their surfaces. Once bound, the interaction causes the shape of one or both to change which transmits a signal. The immune system is heavily dependent upon this type of signaling whereby the immune cells use protein markers to recognize the body’s own cells from pathogens.
Endosymbiotic theory:
With regards to mitochondria, a hypothesis proposed among the scientific community, states that millions of years ago small, free-living prokaryotes were engulfed by larger prokaryotes because they were able to resist the digestive enzymes of the host organism. The two organisms developed a symbiotic relationship over time. The larger organism providing the smaller with ample nutrients and the smaller organism providing ATP molecules to the larger one. Through further evolution, the larger organism developed into the eukaryotic cell and the smaller organism developed into the mitochondrion.
Works Cited:
Caprette, David. “Structure of Mitochondria.” Rice University, 26 May 2005. Web. 9 Feb. 2017.
<http://www.ruf.rice.edu/~bioslabs/studies/mitochondria/mitotheory.html>.
Davidson, M. (2000, October 1). Chloroplasts. Retrieved April 22, 2017, from
https://micro.magnet.fsu.edu/cells/chloroplasts/chloroplasts.html
Davidson, Michael. “Mitochondria.” Molecular Expressions Cell Biology and Microscopy Structure and Function of Cells and Viruses. Florida State University, 1 Oct. 2000. Web. 9 Feb. 2017. <https://micro.magnet.fsu.edu/cells/mitochondria/mitochondria.html>.
Introduction to cell signaling. (n.d.). Retrieved April 22, 2017, from
https://www.khanacademy.org/science/biology/cell-signaling/mechanisms-of-cell-signaling/a/introduction-to-cell-signaling
McClean, P., Ph.D., & Johnson, C. (n.d.). Retrieved April 22, 2017, from
http://vcell.ndsu.nodak.edu/animations/proteintrafficking/transport_02.htm
“Structure Mitochondria.” Tutor Vista, n.d. Web. 9 Feb. 2017. <http://www.tutorvista.com/biology/structure-mitochondria>.
The cytoskeleton. (n.d.). Retrieved April 22, 2017, from
https://www.khanacademy.org/science/biology/structure-of-a-cell/tour-of-organelles/a/the-cytoskeleton
The endomembrane system. (n.d.). Retrieved April 22, 2017, from
https://www.khanacademy.org/science/biology/structure-of-a-cell/tour-of-organelles/a/the-endomembrane-system