

Novel asparaginase activity in nitrile hydratase of Rhodococcus rhodocrous DAP 96253
L-asparaginase also known as L-asparagine amidohydrolase is an important enzyme used to convert L-asparagine to L-aspartate and ammonia. L-asparaginase preparations have been used to treat juvenile Acute Lymphoblastic Leukemia (ALL) for over 40 years. Asparaginase reduces asparagine, depriving leukemia cells of the needed amino acid. In this study, we have shown that the purified nitrile hydratase obtained from specifically induced cells of R. rhodochrous DAP 96253, in addition to exhibiting potent nitrile hydratase activity, also exhibit high levels of L-asparaginase activity.
Research Overview – Asparaginase
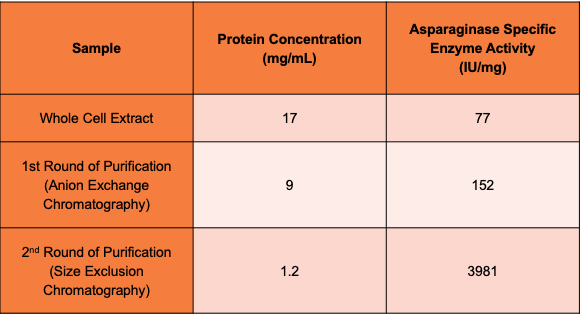
The current standard for producing asparaginase, a treatment for juvenile Acute Lymphoblastic Leukemia (ALL), is recombinant Gram-negative E. coli expression. Removal of endotoxins from products produced through Gram-negative organisms present challenges for proteins used for medical treatment and are required to meet FDA standards. Removal of endotoxin leads to significant loss of product in the manufacturing process. Rhodococcus rhodocrous strain DAP 96253, a Gram-positive soil-based organism, was shown to produce nitrile hydratase with asparaginase activity when induced.
In our preliminary study, nitrile hydratase, purified from induced Rhodococcus rhodochrous DAP 96253, was shown to have asparaginase activity comparable to commercially available asparaginase at physiological temperature.
Jurkat (ATCC- TIB-152) leukemia cell line was used to evaluate asparaginase activity in vitro. Jurkat cell proliferation was slowed and cell survival was greatly reduced over time, with little or no survivorship after 72 hours incubation. Further studies will evaluate purification methods and their effects on asparaginase activity with cells.
