I would like you to bookmark a website that will be helpful for you as you move through biology and biochemistry: Molecular Interactions. This website is a product of the Loren Dean Williams’s lab at Georgia Tech, and is an excellent resource to help you understand the molecular interactions that allow for the structure and function of biomolecules. To start, look at the following sections of the page:
Carbohydrates
While carbohydrates are mainly used as chemical energy storage, carbohydrates are also used as modifiers of proteins and in forming cellular receptors and anchors. One of your goals is to gain a good understanding of the structure of carbohydrates, and a little about their naming.
A topic that will come up throughout the semester is how carbons are numbered in carbohydrates. This is important as we will find carbohydrates being components of monomers and when we move through the carbohydrate catabolism. The following image shows the linear form of glucose, and the two possible cyclic (pyranose ring) isomers.
 |
| http://images.tutorcircle.com/cms/images/44/glucose.png
|
The formation is based on aldehyde chemistry so we will leave some of this discussion to organic chemistry and biochemistry. For our purpose this semester, what is important is that we number carbons from the aldehyde. Notice in the above diagram that carbon 1 is to the left of the oxygen, we go around to carbon 5, and then carbon 6 is outside of the ring. If you see the expression 3′, it is referring to the third carbon. 5′ the fifth carbon. 6′ the sixth carbon, and so forth.
Notice also, that when the ring was formed, there were differences in the groups coming off of carbon 1. These differences are important and can influence how the sugar is metabolized. We say that these different forms are isomers (if you don’t know what an isomer is, look it up and add the definition to your notebook).
One critical difference comes when linking two monosaccharides together to form disaccharides and polysaccharides. For instance, here is maltose:
 |
| https://chemstory.files.wordpress.com/2013/06/dokeo14.png |
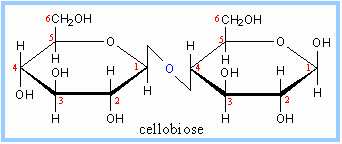
This is an α 1-4 glycosidic linkage. We have an α Maltose (look at carbon 1) bound from carbon 1 to carbon 4. Since the maltose on the left-hand side is α at the 1 carbon, we form an α linkage. In comparison, look at cellobiose:
So, what is the big deal? Maltose is digestible by humans, cellobiose is not. Just this slight isomeric difference changes the metabolism.
All carbohydrate monomers are connected through glycosidic linkages, whether it is a disaccharide, oligosaccharide or a polysaccharide. Make sure that you learn the different types of carbohydrates.
Molecular Visualization
 |
| https://upload.wikimedia.org/wikipedia/commons/8/8a/Alpha-D-Glucose.png |