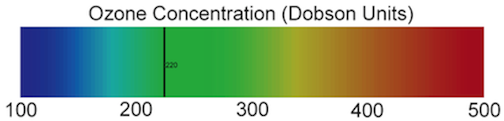
When people hear the word oxygen, they think of the oxygen we breath that keeps us alive. That is one of two forms of oxygen: “regular” oxygen, which is made up of diatomic (two-atom) molecules (O2). There is a second form of oxygen: ozone, which is made up of triatomic (three-atom oxygen) molecules (O3). Ozone is produced in the stratosphere by ultraviolet (UV) radiation splitting a diatomic oxygen (O2) molecule in half. One of the oxygen atoms created by this splitting combines with another O2 molecule to form O3. As you learned in the background information, the stratosphere has the highest concentration of ozone of any of the layers of atmosphere. The total amount of ozone that constitutes the atmosphere is measured in Dobson Units (DU), which is a value determine by measuring the concentration of ozone molecules in a column of air that extends from the Earth’s surface to the top of the atmosphere. Areas with values less than 220 Dobson Units are considered to have experienced severe ozone destruction. The image below presents a color-coded scale for Dobson Units that shows the 220-unit cut-off below which the concentration of ozone is recognized as being low enough that it poses a danger to human health. Please note which colors represent high ozone concentrations and which colors represent low ozone concentrations.
Open 1997_DobsonUnits in Google™ Earth. This is an animation of monthly ozone concentrations (in Dobson Units) during 1997. The same scale that appears above will be embedded in the animation. As you explore the animation use that scale to note changes in the ozone concentration over Atlanta, the Arctic Circle, and Antarctica during the months of 1997. It is important to note that the black areas are areas where ozone concentrations were not measured; these are not holes in the ozone layer.
![]() Q1: What is the range in concentrations of stratospheric ozone during the course of the year over Atlanta? What does this indicate about the amount of change of ozone over the course of the year?
Q1: What is the range in concentrations of stratospheric ozone during the course of the year over Atlanta? What does this indicate about the amount of change of ozone over the course of the year?
![]() Q2: What is the range in concentrations of stratospheric ozone during the course of the year over the Arctic Circle? What does this indicate about the amount of change of ozone over the course of the year?
Q2: What is the range in concentrations of stratospheric ozone during the course of the year over the Arctic Circle? What does this indicate about the amount of change of ozone over the course of the year?
![]() Q3: What is the range in concentrations of stratospheric ozone during the course of the year over Antarctica? What does this indicate about the amount of change of ozone over the course of the year?
Q3: What is the range in concentrations of stratospheric ozone during the course of the year over Antarctica? What does this indicate about the amount of change of ozone over the course of the year?
![]() Q4: What value for the Dobson Units would constitute a true hole in the ozone layer? Based on this answer, did the data from 1997 show a true hole in the ozone layer over any place on Earth?
Q4: What value for the Dobson Units would constitute a true hole in the ozone layer? Based on this answer, did the data from 1997 show a true hole in the ozone layer over any place on Earth?