Neural circuits underlying visual receptive field properties
Starting with my doctoral dissertation, I investigated how visual receptive field properties are constructed in terms of neural circuitry. At that time there was a debate about whether complex properties such as stimulus size and velocity tuning were produced by specialized cell types in parallel pathways, or by convergence of multiple inputs onto a target cell population. By making partial lesions to the developing superior colliculus (SC), I could increase retinal convergence onto SC neurons and test these alternate hypotheses. This body of work demonstrated that specific spatial convergence patterns of inhibitory and excitatory connections are responsible for stimulus size tuning, whereas velocity tuning results from spatial convergence and asymmetric temporal summation.
- Pallas, S.L. M. Gilmour, B.L. Finlay (1988) Control of cell number in the developing neocortex: I. Effects of early tectal ablation. Devel. Brain Res. 43: 1-11. PMID: 2464411
- Razak, K. A., S.L. Pallas (2005) Neural mechanisms of stimulus velocity tuning in the superior colliculus. J. Neurophysiol. 94:3573-3589. PMID: 16079191
- Razak, K. A., S.L. Pallas (2006) Dark rearing reveals the mechanism underlying stimulus size tuning of superior colliculus neurons. Visual Neurosci. 23: 741-748. PMID: 17020630
- Razak, K.A., S.L. Pallas (2007) Inhibitory plasticity facilitates recovery of stimulus velocity tuning in the superior colliculus after chronic NMDA receptor blockade. J. Neurosci. 27: 7275-7283. PMID: 17611280
Stability of visual receptive field properties after injury or activity
The results from the experiments discussed above also showed that compression of the retinal projection onto a partially lesioned SC led to a compensatory process that- while increasing convergence at the population level- somehow preserved single cell convergence ratios, maintaining normal receptive field sizes. Furthermore, size and velocity tuning were preserved. The following papers showed that this occurred by reductions in retinal axon arbor extent and by both NMDA receptor-dependent plasticity and GABAA receptor-dependent inhibitory plasticity.
- Pallas, S.L., B.L. Finlay (1991) Compensation for population size mismatches in the hamster retinotectal system: Alterations in the organization of retinal projections. Visual Neuroscience 6: 271-281. PMID: 2054328
- Xiong, M.-J., S.L. Pallas, S. Lim, B.L. Finlay (1994) Regulation of retinal ganglion cell axon arbor size by target availability: Mechanisms for compression and expansion of the retinotectal projection. J. Comp. Neurol. 344: 581-597. PMID: 7929893
- Huang, L., S.L. Pallas (2001) NMDA antagonists in the superior colliculus prevent developmental plasticity but not visual transmission or map compression. J. Neurophysiol. 86:1179-1194. PMID: 11535668
- Razak, K.A., L. Huang, S.L. Pallas (2003) NMDA receptor blockade in the superior colliculus increases receptive field size without altering velocity and size tuning. J. Neurophysiol. 90:110-119. PMID: 12611963
Development and maintenance of refined visual receptive fields
Decades of research on visual cortex have led to a consensus that visual experience is necessary for normal development of visual pathways. We expected the same finding in superior colliculus, but instead found that refinement of visual projections to SC occurred normally under dark rearing conditions. Surprisingly, vision is necessary only for adult maintenance of refined receptive fields, not only in SC but also in visual cortex. A brief exposure to light during the second postnatal month protects against receptive field enlargement for the life of the animal. No amount of light exposure after this critical period will be able to reverse the detrimental loss of refinement. Plasticity in adulthood, whether adaptive or maladaptive, is increasingly being acknowledged, although the threshold and extent of plasticity is higher in young animals. We are continuing this project with funding from NSF.
- Carrasco, M.M., K.A. Razak, S.L. Pallas (2005) Visual experience is necessary for maintenance but not development of refined retinotopic maps in superior colliculus. J. Neurophysiol. 94: 1962-1970. PMID: 15917326
- Carrasco, M.M., S.L. Pallas (2006) Early visual experience prevents but cannot reverse deprivation-induced loss of refinement in adulthood. Visual Neurosci. 23:845-852. PMID: 17266776
- Carrasco, M.M., Y.-T. Mao, T. Balmer, S.L. Pallas (2011) Inhibitory plasticity underlies visual deprivation-induced loss of retinocollicular map refinement in adulthood. Eur J Neurosci 33:58-68. PMID: 21050281
- Balmer, T.S., S.L. Pallas (2013) Refinement but not maintenance of receptive fields in both superior colliculus and visual cortex is independent of visual experience. Cerebral Cortex 25:904-917. PMID: 24108803
Cross-modal plasticity
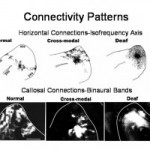
Beginning from my postdoctoral training, I studied cross-modal plasticity of sensory pathways in ferrets. Damage to the dorsal midbrain deafferents inferior colliculus and eliminates SC as a retinal target structure. As a result, retinal axons develop new projections to the auditory thalamus (MGN), thus carrying visual activity patterns to auditory cortex. Our work in the Sur lab demonstrated that auditory cortical neurons receiving visual input via auditory thalamus could support retinotopy, visual response properties, and visual perception. In my own lab, I asked how auditory function would be impacted by the ectopic visual inputs. Surprisingly we found that although auditory responses were still present in the bimodally-innervated auditory cortex, many bisensory neurons were created, intracortical and horizontal connectivity were severely altered, and the tonotopic organization was absent. Early deafened animals also had profound connectivity disturbances and a loss of modular organization as adults. This work suggests that damage to the auditory pathway can lead to maladaptive plasticity at the primary auditory cortical level that could interfere with the success of cochlear implants or other rehabilitative measures.
- Pallas, S.L., T. Littman, D.R. Moore (1999) Cross–modal reorganization of callosal connectivity without altering thalamocortical projections. Natl. Acad. Sci. USA 96: 8751-8756. PMID: 10411947
- Gao, W.-J., S.L. Pallas (1999) Cross-modal reorganization of horizontal connectivity in auditory cortex without altering thalamocortical projections. J. Neurosci. 19:7940-7950. PMID: 10479695
- Mao, Y.-T, T.-M. Hua, S.L. Pallas (2011) Competition and convergence between auditory and cross-modal visual inputs to primary auditory cortical areas. J. Neurophysiol. 105:1558-1573. PMID: 21273321
- Mao, Y.-T, S.L. Pallas (2012) Compensation and compromise of auditory cortical function after invasion by visual inputs. J. Neurosci. 32:10338-10351. PMID: 22836267
Inhibitory plasticity
A common theme began to emerge across my various studies of sensory system plasticity. In each case, plasticity of GABAergic synapses was a major component of the underlying mechanism. Sensory deprivation led to a loss of inhibitory tone that resulted in expansion of receptive fields but also a maintenance of velocity tuning. Cross-modal plasticity resulted in a complex rewiring of inhibitory inputs to support 2-dimensional visual representations. The concept of use-dependent plasticity of inhibitory synapses was understudied and relatively novel, which led me to organize a colloquium and then an edited book on mechanisms of inhibitory plasticity. Inhibitory synaptic plasticity is now accepted as a major contributor to postnatal alterations in sensory circuits.
- Gao, W.-J., D.E. Newman, A.B. Wormington, S.L. Pallas (1999) Development of inhibitory circuitry in visual and auditory cortex of postnatal ferrets: immunocyto-chemical localization of GABAergic neurons. J. Comp. Neurol. 409: 261-273. PMID: 10379919
- Gao, W.J, A.B. Wormington, D.E. Newman, S.L. Pallas (2000) Development of inhibitory circuitry in visual and auditory cortex of postnatal ferrets: immunocytochemical localization of calbindin- and parvalbumin-containing neurons. J Comp Neurol. 422:140-57. PMID: 10842223
- Mao, Y.-T, S.L. Pallas (2013) Cross-modal plasticity results in increased inhibition in primary auditory cortical areas. Neural Plasticity 2013, article ID 530651, doi.org/10.1155/2013/530651 PMID: 24288625
- Balmer, T.S., S.L. Pallas (2015) Visual experience prevents dysregulation of GABAB receptor-dependent short-term depression in adult superior colliculus. J. Neurophysiol. 113: 2049-2061. PMID: 25568162
DATA SHARING POLICY:
Data produced in the Pallas lab may be useful to researchers in the fields of developmental and visual neuroscience. After publication we are willing to share any of the data used to generate our manuscripts. We ask only that the PI(s) and other authors involved in generating the data receive proper attribution.
Catalog of available resources (requests to spallas@gsu.edu):
- Nissl-stained sections
- Adult ferret, hamster, mouse (coronal, some sagittal)
- Developmental series: ferret, hamster, mouse (coronal, some sagittal)
- Immunostained sections
- Ferret: developmental series GABA, Parvalbumin, Calbindin, VIP, Somatostatin
- Hamster: Normally reared and dark-reared, GABA, TrkB, Phospho-TrkB
- In situ hybridization images
- Hamster: developmental series ephrin-A2, ephrin-A5 mRNA
- Tract-tracing images
- Ferret auditory cortex: retrograde, anterograde, local (archival tracers only)
- Electrophysiological recordings
- Digitized files containing background and stimulus-evoked activity from in vivo recordings available for
- Ferret auditory cortical responses to sound and light stimuli
- Hamster and mouse superior colliculus and visual cortex responses to light stimuli
- Digitized files containing background and stimulus-evoked activity from in vivo recordings available for
We will evaluate requests for data by researchers who satisfy the criteria set out by NSF in the Grant Proposal Guide Chapter I Section E for qualified scientists, engineers, and educators. In the case of graduate students requesting access, sponsorship by a faculty member meeting the guidelines for eligibility to submit proposals will be required.
Intellectual property rights are set by Georgia State University Policy No. GSU: 4.00.08. Data will be embargoed only until publication, unless the University requests a delay in public dissemination if necessary to permit the University to secure protection for Intellectual Property disclosed to it by the PI.