Document Review
A document review is a process that entails the collection and interpretation of relevant documents for research purposes. There are various documents that may be reviewed using this method and may vary in format, type, purpose, and its initial development. How document reviews are defined, and its purpose will depend on the industry.
Document reviews can be as a valid resource when conducting research or evaluations with the intent to discover or validate information. In addition to research, document reviews can also be used to verify compliance or ensure certain quality standards are met. It is vital, especially when confirming data or suspensions, that the documents collected are relevant and accurate, as it relates to the topic and/or goals.
Document reviews used for research purposes can aid the discovery or help to determine effective approaches to resolve related challenges or issues.
Though a cost-effective instrument for research, initiating document reviews can prove to be tedious and may require the need for additional skill, knowledge, or time to be of any value.
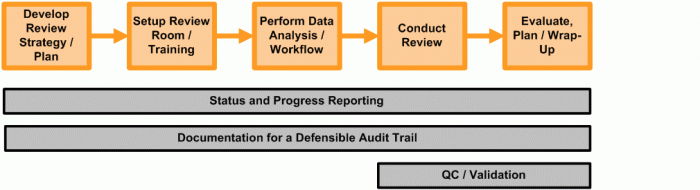
The image below illustrates the process one firm endures as it completes document reviews.
References
Centers for Disease Control and Prevention (2009). Data collection methods for evaluation: Document review (No. 18). Retrieved from website: http://www.cdc.gov/healthyyouth/evaluation/pdf/brief18.pdf
Bowen, G. A. (2009). Document Analysis as a Qualitative Research Method. Qualitative Research Journal, 9(2), 27–40. doi: 10.3316/qrj0902027
Review Guide [Digital image]. (2010). Retrieved April 11, 2019, from https://www.edrm.net/frameworks-and-standards/edrm-model/review-guide/
