Before beginning the lab, please watch the short video below. Mila is going to introduce the topic, show you to download and use Google Earth, and then end the video by reminding you of the three main questions you should be able to answer at the end of the lab.
This lab has 21 short-answer questions you will answer prior to the three big questions (i.e. , research questions) Mila has noted above.
Section 1
In the first lab in this course (Solar Radiation & Seasons), we looked at the effect that the Sun’s radiant energy has on the surface of the Earth – specifically, the global surface temperature – and how this effect was controlled by the intensity and duration of the radiation. We noted in particular how changes in intensity (caused by the fixed tilt of the Earth) are responsible for the seasons. In this lab, we are interested in solar radiation again, but move the scene of our story a few miles upwards from the Earth’s surface to the atmosphere above it. And it turns out that a funny thing happens to that radiation as it passes through the Earth’s atmosphere: Some of it does not make it as it is absorbed by certain gases found in differing amounts in the various layers which comprise the Earth’s atmosphere. This lab will focus on one of those layers – the stratosphere – and one particular gas which has its greatest abundance in this layer – ozone – because of the importance of the type of radiation that is absorbed by the ozone in the stratosphere to the health of each and everyone one of us.”
At the end of this lab, you should be able to answer the following research questions:
-
What is the relationship between solar radiation and stratospheric ozone?
-
How and why has stratospheric ozone changed over time?
-
How and why are concentrations of stratospheric ozone expected to change in the future?
__________________________________________________________________________________________
Entering with the right mindset
Throughout this lab you will be asked to answer some questions. Those questions will come in three different varieties:
![]() Fact-based question →This will be a question with a rather clear-cut answer. That answer will be based on information (1) presented by your instructor, (2) found in background sections, or (3) determined by you from data, graphs, pictures, etc. There is more of an expectation of you providing a certain answer for a question of this type as compared to questions of the other types.
Fact-based question →This will be a question with a rather clear-cut answer. That answer will be based on information (1) presented by your instructor, (2) found in background sections, or (3) determined by you from data, graphs, pictures, etc. There is more of an expectation of you providing a certain answer for a question of this type as compared to questions of the other types.
![]() Synthesis-based question → This will be a question that will require you to pull together ideas from different places in order to give a complete answer. There is still an expectation that your answer will match up to a certain response, but you should feel comfortable in expressing your understanding of how these different ideas fit together.
Synthesis-based question → This will be a question that will require you to pull together ideas from different places in order to give a complete answer. There is still an expectation that your answer will match up to a certain response, but you should feel comfortable in expressing your understanding of how these different ideas fit together.
![]() Hypothesis-based question → This will be a question which will require you to stretch your mind little bit. A question like this will ask you to speculate about why something is the way it is, for instance. There is not one certain answer to a question of this type. This is a more open- ended question where we will be more interested in the ideas that you propose and the justification (‘I think this because . . .’) that you provide.
Hypothesis-based question → This will be a question which will require you to stretch your mind little bit. A question like this will ask you to speculate about why something is the way it is, for instance. There is not one certain answer to a question of this type. This is a more open- ended question where we will be more interested in the ideas that you propose and the justification (‘I think this because . . .’) that you provide.
__________________________________________________________________________________________
Section 2
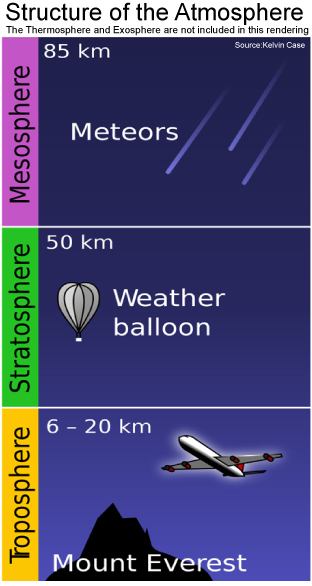
The atmosphere above the Earth is divided into layers (from innermost to outermost: troposphere, stratosphere, mesosphere, thermosphere, and exosphere). The image on the left below shows a profile of the Earth’s atmosphere. We live in the layer of the atmosphere known as the troposphere, which starts at the surface of the Earth and can extend up to 20 kilometers. The next layer is the stratosphere, which is the section found between twenty kilometers and fifty kilometers above the Earth’s surface.
Similar to the troposphere, the stratosphere is comprised almost entirely of nitrogen and oxygen. In contrast to the troposphere, it contains relatively high concentrations of ozone. In fact, the peak ozone concentration occurs between 30 and 35 kilometers above the Earth’s surface, and this is known as the “ozone layer.” Approximately 90% of the ozone in the atmosphere is found in the stratosphere. The middle image below illustrates how the concentration of ozone varies through the different layers of the atmosphere. Even though the concentration of ozone is highest in the stratosphere, ozone still makes up a relatively small percentage of the gases in this layer: only 0.0003 % of the total amount of gas in the stratosphere is ozone. The image on the right below shows you the percentages of several key gases in the stratosphere.
![]() Q1: What layer of the atmosphere is below the stratosphere?
Q1: What layer of the atmosphere is below the stratosphere?
![]() Q2: What gas has a higher concentration in the stratosphere than in the troposphere?
Q2: What gas has a higher concentration in the stratosphere than in the troposphere?
So why are we talking so much about the stratosphere and the ozone in it? To appreciate this, you need to think back to the previous lab when you learned that the radiant energy from the sun is composed of different types of electromagnetic (EM) radiation (visible, ultraviolet or UV, etc.). These different types of radiation are distinguished by their wavelengths and therefore their energies. With its shorter wavelengths (compared to visible light), UV radiation is energetic enough to cause damage to certain things that absorb it – such as your skin (leading to cancer), your eyes (leading to cataracts), and plant leaves (reducing their size). For example, ultraviolet radiation from sun exposure is the primary cause of skin cancer, and there are at least 2,000,000 new cases of skin cancer in the United States each year.
Just as EM radiation is separated into different types based on wavelength and energy, so is UV radiation: There is UV-A (longest wavelengths), UV-B, and UV-C (shortest wavelengths). When the ozone layer is intact, it absorbs 50% of the UV-A radiation, 90% of the UV-B radiation, and all of the UV-C radiation coming from the sun. (See a graph of this on the left below.) Because of this, when you purchase a sunscreen (aka sunblock), you should look for one that offers UV-A and UV-B protection, so that the sunscreen will hopefully absorb the portions of the UV radiation coming from the sun that the ozone in the stratosphere does not. To help you visualize the important role of the ozone in absorbing (as opposed to reflecting) UV radiation, please take a look at the animation below the picture.
![]() Q3: What does the atmosphere do to most of the UV radiation from the Sun? The atmosphere includes the troposphere, stratosphere, mesosphere, thermosphere, and exosphere.
Q3: What does the atmosphere do to most of the UV radiation from the Sun? The atmosphere includes the troposphere, stratosphere, mesosphere, thermosphere, and exosphere.
In the 1920’s, a team of researchers at General Motors Research Corporation lead by Thomas Midgley created the first chlorofluorocarbon (or CFC), which they called Freon. This team showed that the compound (made of carbon, hydrogen, chlorine, and fluorine) would be a safe alternative to the refrigerants available at the time. Later, other CFC’s were prepared, and found use in a wide variety of other places, including aerosol sprays, foams, and fire extinguishers. In the 1970’s, atmospheric chemists Mario Molina and Sherwood Rowland showed that reactions taking place when the normally stable CFC’s hit the lower temperatures and higher amounts of UV radiation in the stratosphere could break them down – and that once they were broken down, they could attack and destroy ozone molecules. An image from the first paper they published on this serious environmental issue appears below. The discovery that the CFC’s – even when present in extremely low concentrations in the stratosphere (as low as 1 in every 2,000 gas molecules) – could have this effect on the ozone layer was shocking to Sherwood and Rowland – and to the rest of the world as well.
The video below shows how evidence for this thinning of the ozone layer – or ozone “hole” – was discovered in 1985.
![]() Q4: What three conditions are needed for CFCs to cause a “hole” in the ozone layer over Antarctica?
Q4: What three conditions are needed for CFCs to cause a “hole” in the ozone layer over Antarctica?
To say that it is unusual for countries across the world to reach agreement on anything is an understatement. So when the countries came together to sign the Montreal Protocol in September 1987 to phase out the production and use of CFCs (and related compounds), and then began executing this international agreement in January 1989, this was a momentous occasion. It demonstrated how serious the issue at stake was: doing nothing and allowing the concentrations of ozone-depleting chemicals to build up could have catastrophic effects on all the living things on this planet. While the Montreal Protocol was an unbelievably important step forward, it is important to note that it will take a while for this legislation to reverse the effect of the CFCs that were released before it was put into place. The table below – which shows the atmospheric lifetimes for a number of ozone-depleting chemicals, or how long it takes for the concentration of one of these compounds to decrease to their normal level – should help you understand why this is the case. (As you examine this table, you should note that CFC-12 – dichlorodifluoromethane – was the first CFC created and was produced in the largest amounts.)
![]() Q5: Why was the Montreal Protocol enacted and when was it signed?
Q5: Why was the Montreal Protocol enacted and when was it signed?
__________________________________________________________________________________________
Section 3
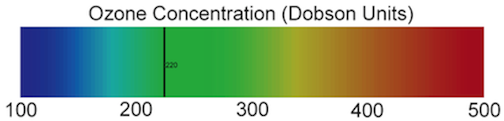
When people hear the word oxygen, they think of the oxygen we breath that keeps us alive. That is one of two forms of oxygen: “regular” oxygen, which is made up of diatomic (two-atom) molecules (O2). There is a second form of oxygen: ozone, which is made up of triatomic (three-atom oxygen) molecules (O3). Ozone is produced in the stratosphere by ultraviolet (UV) radiation splitting a diatomic oxygen (O2) molecule in half. One of the oxygen atoms created by this splitting combines with another O2 molecule to form O3. As you learned in Section 2, the stratosphere has the highest concentration of ozone of any of the layers of atmosphere. The total amount of ozone that constitutes the atmosphere is measured in Dobson Units (DU), which is a value determine by measuring the concentration of ozone molecules in a column of air that extends from the Earth’s surface to the top of the atmosphere. Areas with values less than 220 Dobson Units are considered to have experienced severe ozone destruction. The image below presents a color-coded scale for Dobson Units that shows the 220-unit cut-off below which the concentration of ozone is recognized as being low enough that it poses a danger to human health. Please note which colors represent high ozone concentrations and which colors represent low ozone concentrations.
Open Dobson_Units_1997 in Google™ Earth. This is an animation of monthly ozone concentrations (in Dobson Units) during 1997. The same scale that appears above will be embedded in the animation. As you explore the animation use that scale to note changes in the ozone concentration over Atlanta, the Arctic Circle, and Antarctica during the months of 1997. It is important to note that the black areas are areas where ozone concentrations were not measured. These areas are over the Arctic Cicle and Antarctic Cicle during their respective autumn and winter seasons. The black areas are not holes in the ozone layer.
If you are not using Google™ Earth Pro or prefer to to not use the slider at the top, then you can manually select each month as shown below in the example for January.

![]() Q6: What is the range in concentrations (i.e., minimum to maximum concentrations) of stratospheric ozone during the course of the year over Atlanta? We are using Atlanta as an example of a place in the middle latitudes of the Northern Hemisphere.
Q6: What is the range in concentrations (i.e., minimum to maximum concentrations) of stratospheric ozone during the course of the year over Atlanta? We are using Atlanta as an example of a place in the middle latitudes of the Northern Hemisphere.
![]() Q7: What is the range in concentrations (i.e., minimum to maximum concentrations) of stratospheric ozone during the course of the year over Antarctica?
Q7: What is the range in concentrations (i.e., minimum to maximum concentrations) of stratospheric ozone during the course of the year over Antarctica?
![]() Q8: How much smaller was the minimum concentration over Antarctica compared to the minimum concentrations over Atlanta?
Q8: How much smaller was the minimum concentration over Antarctica compared to the minimum concentrations over Atlanta?
Below is an animation of ozone concentrations over Antarctica from 1979-2004. The data were collected by instruments on NASA satellites. This animation shows total ozone in the Antarctic region along with the maximum ozone layer depth and size since the earliest measurements (from the Earth Probe instrument on the Total Ozone Mapping Spectrometer or TOMS satellite). This animation will represent concentration of ozone using the same color scheme as in the previous animation. Watch the entire animation and keep note of changes in the sizes of the purple and blue areas and occurrences of red areas.
![]() Q9: During what months of the year do the lowest ozone concentrations tend to occur? What season is this for Antarctica? Keep in mind that Antarctica is in the Southern Hemisphere.
Q9: During what months of the year do the lowest ozone concentrations tend to occur? What season is this for Antarctica? Keep in mind that Antarctica is in the Southern Hemisphere.
You learned earlier that the three conditions needed CFCs to be able to efficiently destroy ozone molecules and cause major decreases in ozone concentrations in the stratosphere are as follows: (1) solar radiation, (2) low temperatures, and (3) polar stratospheric clouds.
![]() Q10: Why do you think the months you listed above, as opposed to winter months, had the lowest ozone concentrations?
Q10: Why do you think the months you listed above, as opposed to winter months, had the lowest ozone concentrations?
Moving about 12o in latitude north from the edge of Antarctica, one runs into Tierra del Fuego, an archipelago located off the tip of the South American mainland. To become familiar with the location of this landmass, click on the Google Earth™ file Tierra_del_Fuego. Take the time to note its relative distance from Antarctica, and think about this in relation to the change in ozone concentrations for Antarctica you explored above.
One of the plants native to Tierra del Fuego is Gunnera magellanica, a creeping, flowering species that is fairly hardy. In 1997 a group of researchers lead by Rousseaux studied samples of this plant from across the archipelago to determine what effects on living organisms might have been caused by a migration of the ozone “hole” (hereafter referred to as the severe destruction zone or SDZ) over that part of the Earth. This research particularly examined the damage to the DNA of Gunnera magellanica caused by the increase in UV radiation produced by the migration of the SDZ; click on the image below to see the opening page of the paper written by Rousseaux et al. about their work.
To consider for yourself the relationship between changes in the ozone concentration above Tierra del Fuego and the health of organisms living on it that Rousseaux and his colleagues were studying, first look at the animation below. It shows the changing ozone levels across the lower part of the southern hemisphere from 11 October 1997 to 16 October 1997, using data collected by instruments on NASA satellites. The ozone concentrations are represented using the same color scheme you have seen in the previous animations (the image below is available to remind you of that color scheme). As you look at the animation, try to note the precise timing when the levels of ozone over Tierra del Fuego were at their lowest.
![]() Q11: Based on what you observed in the animation, on what day(s) did the severe destruction zone (i.e., the ozone “hole”) migrate over Tierra del Fuego?
Q11: Based on what you observed in the animation, on what day(s) did the severe destruction zone (i.e., the ozone “hole”) migrate over Tierra del Fuego?
Next, you will look at some of the data from the paper in which Rousseaux et al. discuss their findings. The figure below presents one section from that paper which contains, in order from top to bottom, images of the ozone concentration over Tierra del Fuego for October 14th and October 17th, 1997 (unfortunately, using a different color scheme than you seen in the previous animations); a graph showing the change in UV radiation reaching the surface of the Earth at Tierra del Fuego on those dates; and a graph on DNA damage measured in the Gunnera magellanica samples on those dates. Please answer the questions below the image concerning the relationships between these different things.
![]() Q12: How did the change in ozone concentrations affect the amount of UV radiation reaching the Earth’s surface?
Q12: How did the change in ozone concentrations affect the amount of UV radiation reaching the Earth’s surface?
![]() Q13: What affect did the change in UV radiation have on the vegetation?
Q13: What affect did the change in UV radiation have on the vegetation?
![]() Q14: Based on your answers to Q12 and Q13 and the data you examined, explain whether it would be reasonable for a scientist to state that, “Significant decreases in ozone concentration over a part of the Earth can cause serious damage to plant and animal DNA.”
Q14: Based on your answers to Q12 and Q13 and the data you examined, explain whether it would be reasonable for a scientist to state that, “Significant decreases in ozone concentration over a part of the Earth can cause serious damage to plant and animal DNA.”
__________________________________________________________________________________________
Section 4
Click AntarcticaOzone_Data to open the file in Microsoft® Excel. This file contains yearly values for spring ozone concentration (in Dobson Units) over Antarctica and the size of the severe-destruction zones, which consists of values less than 220 Dobson Units, over Antarctica during spring. Its size is measured in millions of square kilometers. All data are derived from satellite measurements and were obtained from NASA’s Ozone Hole Watch.
You are going to convert that data into a graph within Excel using the following steps:
- Select cells in rows 1 through 43 of columns A, B, and C.
- Under the Insert tab, select Line
- Under the 2-D line options, click on first choice.
The resulting graph shows yearly values of the minimum ozone concentration (upper in blue) and the area of the severe-destruction zone (or Hole Area, lower in red).
- In order to better see the red line (Hole Area), right-click on that line, select Format Data Series, and then change the axis to the Secondary Axis.
The graph will now have the Ozone Concentration (in Dobson Units) on the left vertical axis and will have Hole Area (in square kilometers) on the right vertical axis. Reformatting the graph as you just did should allow you to better see the relationship between the two. Feel free to play around with the graph to make it larger, wider, etc.
![]() Q15: How would you describe the trends in spring ozone concentrations and the “hole” area over Antarctica from 1979 to 1989?
Q15: How would you describe the trends in spring ozone concentrations and the “hole” area over Antarctica from 1979 to 1989?
Hint: They have opposite trends.
![]() Q16: Based on your answer to Q16 (and based on what you see for the entire graph), what is the relationship between Ozone Concentration and Hole Area?
Q16: Based on your answer to Q16 (and based on what you see for the entire graph), what is the relationship between Ozone Concentration and Hole Area?
![]() Q17: How would you describe the trends in spring ozone concentrations and the “hole” area over Antarctica from 1989 to 2020? You are describing what has happened to ozone concentrations and the size of the ozone “hole” since the Montreal Protocol went into force.
Q17: How would you describe the trends in spring ozone concentrations and the “hole” area over Antarctica from 1989 to 2020? You are describing what has happened to ozone concentrations and the size of the ozone “hole” since the Montreal Protocol went into force.
![]() Q18: Based on your analysis of the graph, what effect has the Montreal Protocol had on the stratospheric ozone layer?
Q18: Based on your analysis of the graph, what effect has the Montreal Protocol had on the stratospheric ozone layer?
Notice how the Hole Area has leveled off but has not decreased significantly since the Montreal Protocol was put in place.
![]() Q19: Why is it taking a relatively long time for the “hole” area to decrease in size? Hint: You should inspect a table located above Q5.
Q19: Why is it taking a relatively long time for the “hole” area to decrease in size? Hint: You should inspect a table located above Q5.
Hopefully you also see in your graph that between 1987 and 1988 and then again between 2001 and 2002 and between 2018 and 2019, there were very dramatic decreases in the Hole Area (or severe destruction zone). The ozone hole area was relatively small in 1998, 2002, and 2019 because there were fewer polar stratospheric clouds during those years. Remember that those clouds are needed for the destruction of the ozone layer.
__________________________________________________________________________________________
Section 5
Below is a NASA animation which shows the ozone concentrations over the western hemisphere from 1979 to 2065. The concentration of ozone will be represented using the same Dobson Unit scale that you have seen before. (Just in case you have forgotten the scale is shown below.) The visualizations presents two scenarios: (1) “Projected,” which assumes the current rate of CFC emissions in the atmosphere; and (2) “World Avoided,” where the rate of CFC emissions is assumed to be that of the period before regulation. Therefore, “World Avoided” has much higher rates of CFC emissions than does “Projected” from 1989 to 2065. Watch the entire animation and pay particular attention to areas of low ozone concentration just to the east of the southeastern United States (i.e. over the far western North Atlantic Ocean).
![]() Q20: For the entire visible area of the animation, why do the two scenarios have similar results in the 1970s and 1980s?
Q20: For the entire visible area of the animation, why do the two scenarios have similar results in the 1970s and 1980s?
![]() Q21: What effect should the Montreal Protocol have on the stratospheric ozone layer in future decades?
Q21: What effect should the Montreal Protocol have on the stratospheric ozone layer in future decades?
__________________________________________________________________________________________
Section 6
Before the next lab, write for yourself a one-sentence response to each of the following big questions of this lab.
What is the relationship between solar radiation and stratospheric ozone?
How and why has the stratospheric ozone layer changed over time?
How and why are concentrations of stratospheric ozone expected to change in the future?