Current Research Interests:
1) Control of lipid metabolism by the sympathetic nervous system and sensory innervation of white adipose tissue.
2) Control of thermogenesis by the sympathetic nervous system innervation of brown adipose tissue.
3) Environmental, neural and hormonal control of food acquisition (foraging and hoarding).
View a list of our publications on PUBMED
One goal of our research program is to study the mechanisms underlying the melatonin-induced changes in body fat in Siberian hamsters. This goal is being accomplished through the use of in vivo endocrine/neuroendocrine depletion-repletion paradigms, and through in vitro incubation of isolated adipocytes (fat cells) treated with putative mediators of the melanin-induced lipid met abolic responses. We also are investigating the regulation of seasonally appropriate total body fat content using surgical fat pad removal (lipectomy). In addition, using antero- and retrograde tract tracing techniques and neurotransmitter turn-over measurement, we are continuing to explore our finding that adipocytes are innervated by the sympathetic nervous system (see photograph below). We also are identifying the brain structures that are involved in the reception/interpretation of the melatonin signal by either using selective lesion techniques (e.g., microknife cuts) to disrupt the putative melatonin signal reception system or by stimulating these sites with microinfusions of the hormone. Finally, we are studying the acquisition of food (foraging) and its storage (food hoarding) in a seminatural environment. The goal of these studies is to determine the neural and hormonal mechanisms underlying this naturally occurring behavior including the effects of pregnancy and lactation, as well.
abolic responses. We also are investigating the regulation of seasonally appropriate total body fat content using surgical fat pad removal (lipectomy). In addition, using antero- and retrograde tract tracing techniques and neurotransmitter turn-over measurement, we are continuing to explore our finding that adipocytes are innervated by the sympathetic nervous system (see photograph below). We also are identifying the brain structures that are involved in the reception/interpretation of the melatonin signal by either using selective lesion techniques (e.g., microknife cuts) to disrupt the putative melatonin signal reception system or by stimulating these sites with microinfusions of the hormone. Finally, we are studying the acquisition of food (foraging) and its storage (food hoarding) in a seminatural environment. The goal of these studies is to determine the neural and hormonal mechanisms underlying this naturally occurring behavior including the effects of pregnancy and lactation, as well.
Collectively, these experiments should prove useful in understanding the neural and hormonal control of energy balance and their consequences for understanding pathological conditions of energy balance such as obesity. In addition, we should gain a better understanding of basic fat cell metabolism and the general abilities of animals to make anticipatory adaptive responses that enhance their survival and reproduction. as neuropeptides traditionally tested for their effects on food intake in other species.
The sympathetic nervous system innervates white adipose tissue where it is involved in the mobilization of stored lipid fuels (lipolysis) and innervates brown adipose tissue where it is involved in increasing thermogenesis (generating heat). In some situations, such as cold exposure, both white and brown adipose tissue are stimulated such that needed energy if mobilized from white adipose tissue and brown adipose tissues generates heat.
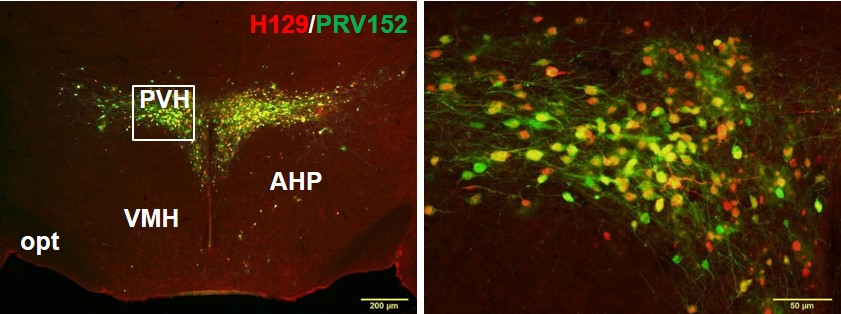 Figure Caption: Double-label fluorescence immunohistochemistry of the paraventricular hypothalamus showing single neurons that are part of the sympathetic outflow to fat (green) using pseudorabies virus (PRV), single neurons part of the sensory inflow to the brain from fat using the H129 strain of the Herpes Simplex Virus 1 (H129) and individual neurons that are part of the sympathetic outflow from brain to white fat that also receive sensory input from white fat (PRV+H129; yellow-orange). Thus, these neurons represent complete neural sympathetic-sensory feedback loops suggesting likely regulation of the lipolysis (fat breakdown) by the sympathetic nerves with feedback as to the degree of lipolysis.
Figure Caption: Double-label fluorescence immunohistochemistry of the paraventricular hypothalamus showing single neurons that are part of the sympathetic outflow to fat (green) using pseudorabies virus (PRV), single neurons part of the sensory inflow to the brain from fat using the H129 strain of the Herpes Simplex Virus 1 (H129) and individual neurons that are part of the sympathetic outflow from brain to white fat that also receive sensory input from white fat (PRV+H129; yellow-orange). Thus, these neurons represent complete neural sympathetic-sensory feedback loops suggesting likely regulation of the lipolysis (fat breakdown) by the sympathetic nerves with feedback as to the degree of lipolysis.
Brown adipose tissue also has sensory system (SS) innervation. At the tissue level, IBAT has immunoreactivity (IR) for two proven SS neuropeptides, substance P and calcitonin gene-related peptide (Norman et al., 1988; De Matteis et al., 1998). Furthermore, with intra-IBAT injection of an anterograde transneuronal viral tract tracer, the H129 strain of herpes simplex virus 1 (HSV-1), we identified the central SS circuits from this tissue (Vaughan and Bartness, 2012). Many of the sites receiving SS input from IBAT (Vaughan and Bartness, 2012) also are part of the SNS outflow from the CNS to IBAT revealed by our PRV studies (Bamshad et al., 1999; Song et al., 2008). Thus, this SNS–SS overlap between studies suggests the possibility of individual neurons that are part of the SNS outflow from brain to BAT that also receive SS input from the tissue; that is, potential SNS–SS feedback loops serving an anatomical basis for crosstalk between BAT SNS and SS innervations. Although the exact role of the BAT SS is unknown, the impairment of the thermogenic response of BAT to acute cold exposure due to sensory denervation accomplished via intra-IBAT injection of the sensory-specific neurotoxin capsaicin (Jancśo et al., 1980, 1985), strongly suggests BAT SS innervation is necessary for its optimal physiological functioning (Vaughan and Bartness, 2012). Therefore, we hypothesized that BAT SNS–SS feedback loops might exist and tested this, as well as identifying which dorsal root ganglia (DRG) receive sensory input when IBAT is adrenergically activated by: (1) injecting both the SS-specific transneuronal tract tracer, H129, and the SNS-specific transneuronal tract tracer, PRV, intra-IBAT to test for dually infected SNS–SS neurons; (2) injecting CL316,243 intra-IBAT using c-Fos-IR to reveal activation (Hoffman et al., 1993) of pseudounipolar neurons within the DRG prelabeled with Fast Blue (FB), thereby identifying IBAT afferent neurons activated by IBAT adrenergic stimulation; and (3) measuring IBAT sensory nerve activity electrophysiologically after intra-IBAT CL316,243 injection. [Excerpt from Ryu et al 2015, J Neurosci]
Figure Caption: Representative microphotographs of DRG at C4 vertebral level showing c-Fos immunostaining (A, D), FB labeling (B, E), and c-Fos+FB colocalization (C, F, arrows) after CL316,243 microinjections intra-left and saline intra-right IBAT. Distribution of c-Fos-IR and FB-labeled neurons in IBAT innervating DRG at C1–T4 vertebral levels contralateral (G) and ipsilateral (H) to CL316,243 microinjections. The total number of positively stained neurons per ganglion can be estimated by multiplying the number per section by 24; n = 12; *p < 0.05 versus saline counter-mate. Scale bar, 50 μm. I, Mean nerve activity from IBAT afferent fibers before (baseline), at 10 and at 20 min after CL316,243 or saline microinjections; n = 5 per group. CL316,243-evoked increase in IBAT sensory nerve activity. Bottom, Ten minute sample traces of IBAT afferent fibers before and after infusion of either CL316,243 (top) or saline (bottom); *p < 0.05 versus saline.
Mechanisms of food hoarding and foraging in Siberian hamsters
Figure Caption: Two cages are positioned one above the other and are connected by approximately 1.52 m tubing that has corners and straightways for both horizontal and vertical climbs. The bottom cage represents an underground burrow, is dark and contains bedding and nesting material. The top cage represents an above-ground foraging area, is lit and contains a running wheel and a water bottle. Food pellets (75 mg) are presented contingent upon the completion of a programmed number of wheel revolutions in the upper cage.
“The study of ingestive behaviour has an extensive history, starting as early as 1918 when Wallace Craig, an animal behaviourist, coined the terms ‘appetitive’ and ‘consummatory’ for the two-part sequence of eating, drinking and sexual behaviours. Since then, most ingestive behaviour research has focused on the neuroendocrine control of food ingestion (consummatory behaviour). The quantity of food eaten, however, is also influenced by the drive both to acquire and to store food (appetitive behaviour). For example, hamster species have a natural proclivity to hoard food and preferentially alter appetitive ingestive behaviours in response to environmental changes and/or metabolic hormones and neuropeptides, whereas other species would instead primarily increase their food intake. Therefore, with the strong appetitive component to their ingestive behavior that is relatively separate from their consummatory behaviour, they seem an ideal model for elucidating the neuroendocrine mechanisms underlying the control of food hoarding and foraging” (Teubner, Bartness, 2010).
“We have focused on definin g the mechanisms underlying the impressive increases in food hoarding and foraging that accompany refeeding after food deprivation [15,35–37]. To date, we have shown that food deprivation increases circulating concentrations of the active form of ghrelin, the largely stomach-derived peptide that stimulates food intake in laboratory rats and mice [34]. Ghrelin, in turn, appears to stimulate its receptors (growth hormone secretagogue receptors) some of which are located in the arcuate nucleus of the brain [60] on NPY/AgRP neurons [10,33]. These arcuate nucleus neurons send projections to several brain areas including the hypothalamic paraventricular nucleus (PVH) and perifornical area (PFA) [22,38]. Both areas possess NPY receptors [46,47] and parenchymal microinjections of NPY into either area stimulates food hoarding [15] that is reminiscent of the increases in food hoarding seen after systemic ghrelin injections in non-food-deprived hamsters [34] or after 3rd ventricular injections of NPY [19]. Although this work is far from complete, we have not as actively pursued the factors that terminate food foraging and hoarding.” [see Keen-Rhinehart, Dailey, Bartness (2010) for references]
g the mechanisms underlying the impressive increases in food hoarding and foraging that accompany refeeding after food deprivation [15,35–37]. To date, we have shown that food deprivation increases circulating concentrations of the active form of ghrelin, the largely stomach-derived peptide that stimulates food intake in laboratory rats and mice [34]. Ghrelin, in turn, appears to stimulate its receptors (growth hormone secretagogue receptors) some of which are located in the arcuate nucleus of the brain [60] on NPY/AgRP neurons [10,33]. These arcuate nucleus neurons send projections to several brain areas including the hypothalamic paraventricular nucleus (PVH) and perifornical area (PFA) [22,38]. Both areas possess NPY receptors [46,47] and parenchymal microinjections of NPY into either area stimulates food hoarding [15] that is reminiscent of the increases in food hoarding seen after systemic ghrelin injections in non-food-deprived hamsters [34] or after 3rd ventricular injections of NPY [19]. Although this work is far from complete, we have not as actively pursued the factors that terminate food foraging and hoarding.” [see Keen-Rhinehart, Dailey, Bartness (2010) for references]

